The animal and its food
Food is material that, after ingestion by animals, is capable of being digested, absorbed and utilised. In a more general sense we use the term ‘food’ to describe edible material. Grass and hay, for example, are described as foods, but not all their components are digestible. Where the term ‘food’ is used in the general sense, as in this book, those components capable of being utilised by animals are described as nutrients. The animals associated with humans cover the spectrum from herbivores, the plant eaters (ruminants, horses and small animals such as rabbits and guinea pigs); through omnivores, which eat all types of food (pigs and poultry); to carnivores, which eat chiefly meat (dogs and cats). Under the control of humans these major classes of animal still pertain, but the range of foods that animals are now offered is far greater than they might normally consume in the wild (for example, ruminants are given plant by-products of various human food industries and some dog foods contain appreciable amounts of cereals). Nevertheless, plants and plant products form the major source of nutrients in animal nutrition. The diet of farm animals in particular consists of plants and plant products, although some foods of animal origin such as fishmeal and milk are used in limited amounts. Animals depend upon plants for their existence and consequently a study of animal nutrition must necessarily begin with the plant itself. Plants are able to synthesise complex materials from simple substances such as carbon dioxide from the air, and water and inorganic elements from the soil. By means of photosynthesis, energy from sunlight is trapped and used in these synthetic processes. The greater part of the energy, however, is stored as chemical energy within the plant itself and it is this energy that is used by the animal for the maintenance of life and synthesis of its own body tissues. Plants and animals contain similar types of chemical substances, and we can group these into classes according to constitution, properties and function. The main components of foods, plants and animals are:
WATER
The water content of the animal body varies with age.The newborn animal contains
750–800 g/kg water but this falls to about 500 g/kg in the mature fat animal. It is
vital to the life of the organism that the water content of the body be maintained: an
animal will die more rapidly if deprived of water than if deprived of food. Water
functions in the body as a solvent in which nutrients are transported about the body
and in which waste products are excreted. Many of the chemical reactions brought
about by enzymes take place in solution and involve hydrolysis. Because of the high
specific heat of water, large changes in heat production can take place within the
animal with very little alteration in body temperature. Water also has a high latent
heat of evaporation, and its evaporation from the lungs and skin gives it a further
role in the regulation of body temperature.
The animal obtains its water from three sources: drinking water, water present in its
food, and metabolic water, this last being formed during metabolism by the oxidation of
hydrogen-containing organic nutrients. The water content of foods is variable and can
range from as little as 60 g/kg in concentrates to over 900 g/kg in some root crops. Because of this great variation in water content, the composition of foods is often expressed on a dry matter basis, which allows a more valid comparison of nutrient content.
This is illustrated inTable 1.1, which lists a few examples of plant and animal products.
The water content of growing plants is related to the stage of growth, being greater
in younger plants than in older plants. In temperate climates the acquisition of drinking water is not usually a problem and animals are provided with a continuous supply.
There is no evidence that under normal conditions an excess of drinking water is
harmful, and animals normally drink what they require.
 |
| Table 1.1 Composition of some plant and animal products expressed on a fresh basis and a dry matter basis |
DRY MATTER AND ITS COMPONENTS
The dry matter (DM) of foods is conveniently divided into organic and inorganic material, although in living organisms there is no such sharp distinction. Many organic
compounds contain mineral elements as structural components. Proteins, for example, contain sulphur, and many lipids and carbohydrates contain phosphorus.
It can be seen from Table 1.1 that the main component of the DM of pasture grass
is carbohydrate, and this is true of all plants and many seeds. The oilseeds, such as
groundnuts, are exceptional in containing large amounts of protein and lipid material. In contrast, the carbohydrate content of the animal body is very low. One of the
main reasons for the difference between plants and animals is that, whereas the cell
walls of plants consist of carbohydrate material, mainly cellulose, the walls of animal
cells are composed almost entirely of lipid and protein. Furthermore, plants store
energy largely in the form of carbohydrates such as starch and fructans, whereas an
animal’s main energy store is in the form of lipid.
The lipid content of the animal body is variable and is related to age, the older animal containing a much greater proportion than the young animal.The lipid content of
living plants is relatively low, that of pasture grass, for example, being 40–50 g/kg DM.
In both plants and animals, proteins are the major nitrogen-containing compounds. In plants, in which most of the protein is present as enzymes, the concentration is high in the young growing plant and falls as the plant matures. In animals,
muscle, skin, hair, feathers, wool and nails consist mainly of protein.
Like proteins, nucleic acids are also nitrogen-containing compounds and they
play a basic role in the synthesis of proteins in all living organisms. They also carry
the genetic information of the living cell.
The organic acids that occur in plants and animals include citric, malic, fumaric,
succinic and pyruvic acids. Although these are normally present in small quantities,
they nevertheless play an important role as intermediates in the general metabolism
of the cell. Other organic acids occur as fermentation products in the rumen, or in
silage, and these include acetic, propionic, butyric and lactic acids.
Vitamins are present in plants and animals in minute amounts, and many of them
are important as components of enzyme systems. An important difference between
plants and animals is that, whereas the former can synthesise all the vitamins they
require for metabolism, animals cannot, or have very limited powers of synthesis,
and are dependent upon an external supply.
The inorganic matter contains all those elements present in plants and animals
other than carbon, hydrogen, oxygen and nitrogen. Calcium and phosphorus are the
major inorganic components of animals, whereas potassium and silicon are the main
inorganic elements in plants.
ANALYSIS AND CHARACTERISATION OF FOODS
Originally the most extensive information about the composition of foods was based
on a system of analysis described as the proximate analysis of foods, which was
devised over 100 years ago by two German scientists, Henneberg and Stohmann.
More recently, new analytical techniques have been introduced, and the information
about food composition is rapidly expanding (see below). However, the system of proximate analysis still forms the basis for the statutory declaration of the composition of foods in Europe.
Proximate analysis of foods
This system of analysis divides the food into six fractions: moisture, ash, crude protein, ether extract, crude fibre and nitrogen-free extractives.
The moisture content is determined as the loss in weight that results from drying a
known weight of food to constant weight at 100 °C.This method is satisfactory for most
foods, but with a few, such as silage, significant losses of volatile material (short-chain
fatty acids and alcohols) may take place.Therefore, for silages, the moisture content can
be determined directly by distilling the water from the sample under toluene.The distillate is measured and corrected for the presence of fermentation acids and alcohols.
The ash content is determined by ignition of a known weight of the food at 550 °C
until all carbon has been removed. The residue is the ash and is taken to represent
the inorganic constituents of the food. The major component of ash is silica but ash
may, however, contain material of organic origin such as sulphur and phosphorus
from proteins, and some loss of volatile material in the form of sodium, chloride,
potassium, phosphorus and sulphur will take place during ignition.The ash content
is thus not truly representative of the inorganic material in the food either qualitatively or quantitatively. Animals do not have a requirement for ash per se but require the individual mineral elements that it contains and are determined by
methods such as atomic absorption spectrometry (see p. 12).
The crude protein (CP) content is calculated from the nitrogen content of the food,
determined by a modification of a technique originally devised by Kjeldahl over
100 years ago. In this method the food is digested with sulphuric acid, which converts
to ammonia all nitrogen present except that in the form of nitrate and nitrite. This
ammonia is liberated by adding sodium hydroxide to the digest, distilled off and
collected in standard acid, the quantity so collected being determined by titration or
by an automated colorimetric method. It is assumed that the nitrogen is derived from
protein containing 16 per cent nitrogen, and by multiplying the nitrogen figure by 6.25
(i.e. 100/16) an approximate protein value is obtained.This is not ‘true protein’ since
the method determines nitrogen from sources other than protein, such as free amino
acids, amines and nucleic acids, and the fraction is therefore designated crude protein.
The ether extract (EE) fraction is determined by subjecting the food to a continuous extraction with petroleum ether for a defined period.The residue, after evaporation of the solvent, is the ether extract. As well as lipids it contains organic acids,
alcohol and pigments.This procedure is referred to as method A. In the current official
method, the extraction with ether is preceded by hydrolysis of the sample with sulphuric acid and the resultant residue is the acid ether extract (method B).
The carbohydrate of the food is contained in two fractions, the crude fibre (CF)
and the nitrogen-free extractives (NFE).The former is determined by subjecting the
residual food from ether extraction to successive treatments with boiling acid and
alkali of defined concentration; the organic residue is the crude fibre.
When the sum of the amounts of moisture, ash, crude protein, ether extract and
crude fibre (expressed in g/kg) is subtracted from 1000, the difference is designated the
nitrogen-free extractives.The nitrogen-free extractives fraction is a heterogeneous mixture of all those components not determined in the other fractions. The crude fibre
fraction contains cellulose, lignin and hemicelluloses, but not necessarily the whole amounts of these that are present in the food: a variable proportion of the cell wall
material, depending upon the species and stage of growth of the plant material, is
dissolved during the crude fibre extraction and thus is contained in the nitrogen-free
extractives. This leads to an underestimation of the fibre and an overestimation of
the starch and sugars. Thus the nitrogen-free extractive fraction includes starch,
sugars, fructans, pectins, organic acids and pigments, in addition to those components
mentioned above.
Modern analytical methods
In recent years the proximate analysis procedure has been severely criticised by
many nutritionists as being archaic and imprecise, and in the majority of laboratories
it has been partially replaced by other analytical procedures. Most criticism has been
focused on the crude fibre, ash and nitrogen-free extractives fractions for the reasons
described above. The newer methods have been developed to characterise foods in
terms of the methods used to express nutrient requirements. In this way, an attempt
is made to use the analytical techniques to quantify the potential supply of nutrients
from the food. For example, for ruminants, analytical methods are being developed
that describe the supply of nutrients for the rumen microbes and the host digestive
enzyme system (Fig. 1.1).
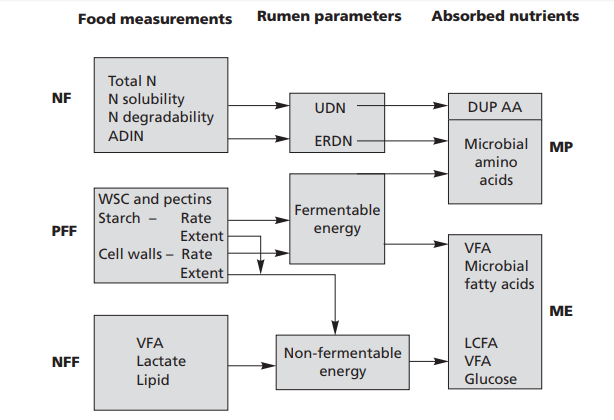 |
| Fig. 1.1 Proposed model for characterisation of foods for ruminants. |
AA = amino acids, ADIN = acid detergent insoluble nitrogen, DUP = digestible undegradable
protein, ERDN = effective rumen degradable nitrogen, LCFA = long-chain fatty acids,
ME = metabolisable energy, MP = metabolisable protein, N = nitrogen, NF = nitrogen fraction,
NFF = non-fermentable fraction, PFF = potentially fermentable fraction, UDN = undegradable
nitrogen, VFA = volatile fatty acids, WSC = water-soluble carbohydrates.
From Agricultural and Food Research Council 1998 Technical Committee on Responses to Nutrients, report
no. 11, Wallingford, CABI.
Starch and sugars
Inadequacies in the nitrogen-free extractives fraction have been addressed by the
development of methods to quantify the non-structural carbohydrates, which are
mainly starches and sugars. Sugars can be determined colorimetrically after combination with a reagent such as anthrone. Starch is determined by dilute acid hydrolysis of the sample followed by polarimetric determination of the released sugars.
This gives a figure for total sugars (i.e. those originating from the hydrolysed starch
plus the simple sugars in the food). Sugars per se are determined by extracting the
sample with ethanol, acidifying the filtrate and taking a second polarimeter reading. The starch content is calculated from the difference between the two readings
multiplied by a known factor for the starch source. Starch can also be determined
enzymically. For example, in cereals starch is converted to glucose using -amylase
followed by amyloglucosidase and then the glucose is measured using the glucose
oxidase-peroxidase reagent.
Fibre
Alternative procedures for fibre have been developed by Van Soest (Table 1.2). The
neutral-detergent fibre (NDF), which is the residue after extraction with boiling neutral solutions of sodium lauryl sulphate and ethylenediamine tetraacetic acid (EDTA),
consists mainly of lignin, cellulose and hemicellulose and can be regarded as a measure of the plant cell wall material. The analytical method for determining NDF was
originally devised for forages, but it can also be used for starch-containing foods provided that an amylase treatment is included in the procedure. By analogy with the
nitrogen-free extractives fraction discussed above, the term non-structural carbohydrate (NSC) is sometimes used for the fraction obtained by subtracting the sum of
the amounts (g/kg) of CP, EE, ash and NDF from 1000.
The acid-detergent fibre (ADF) is the residue after refluxing with 0.5 M sulphuric
acid and cetyltrimethyl-ammonium bromide, and represents the crude lignin and
cellulose fractions of plant material but also includes silica.
 |
| Table 1.2 Classification of forage fractions using the detergent methods of Van Soest |
The determination of ADF is particularly useful for forages as there is a good statistical correlation between it and the extent to which the food is digested (digestibility). In the UK the ADF method has been modified slightly, the duration of boiling
and acid strength being increased.The term modified acid-detergent fibre (MADF) is
used to describe this determination.
The acid-detergent lignin determination involves the preparation of aciddetergent fibre as the preparatory step. The ADF is treated with 72 per cent sulphuric acid, which dissolves cellulose.Ashing the residue determines crude lignin,
including cutin.
The Van Soest methods of fibre analysis are used in the system of food analysis
for ruminants developed at Cornell University (see Box 1.1).
In monogastric, and particularly human, nutrition the term dietary fibre is often
used and attention has been focused on its importance in relation to health. Dietary
fibre (DF) was defined as lignin plus those polysaccharides that cannot be digested
by monogastric endogenous enzymes. Initially epidemiological studies linked a lack
of DF to constipation, gut and bowel disorders, cardiovascular disease and type 2
diabetes; however, the causes of such diseases are multifactorial and in some cases
it is not just DF per se that has the beneficial effects but other aspects of the diet
also (e.g. antioxidants). Nevertheless, DF is a major component related to health in
humans and it has equally important effects in animals (see below).
The definition of DF has proved difficult, with definitions ranging through
physiological/botanical (derived from cell walls of plants, which are poorly digested);
chemical/botanical (non-starch polysaccharides (NSP) of plant cell walls); chemical (NSP and lignin); and nutritional/physiological (NSP not digested in the small
intestine). The common features of DF definitions are carbohydrates (polysaccharides, oligosaccharides and lignin) resistant to digestion in the small intestine but
that may be fermented in the large intestine and promote beneficial physiological effects. By virtue of its definition, DF is difficult to determine in the laboratory. The NSP in most foods, along with lignin, are considered to represent the
major components of cell walls. Methods for measurement of NSP fall into two
 |
| BOX 1.1 The Cornell net carbohydrate and protein system |
categories (with slight variations in the second category, depending on the research laboratory):
- Enzymic–gravimetric methods, which measure a variety of components and give no details of polysaccharide type. In the method of the Association of Official Analytical Chemists for total dietary fibre, samples are gelatinised by heating and treated with enzymes to remove starch and proteins.The total dietary fibre is precipitated with ethanol and the residue is dried and weighed.
- Enzymic–chromatographic methods, which identify the individual carbohydrates in the dietary NSP. The Englyst method can be used to determine total, soluble and insoluble dietary fibre. Measurement of NSP by this method involves removal of starch with the enzymes pullulanase and -amylase. After precipitation with ethanol, the NSP residue is then hydrolysed with 12 M sulphuric acid. The individual monomeric neutral sugar constituents are determined by gas–liquid chromatography (see below) with separate determination of uronic acids. Alternatively, the total sugars are determined colorimetrically after reaction with dinitrosalicylate solution. Total NSP and insoluble NSP are determined directly by analysis of separate subsamples and the soluble NSP are calculated by difference. The major constituents of NSP are rhamnose, arabinose, xylose, glucose, galactose, mannose and glucuronic and galacturonic acids. Cellulose is the major source of glucose, and hemicellulose provides xylose, mannans and galactose.The degradation of pectins releases arabinose, galactose and uronic acids. Following the adoption of methods to determine NSP, it became apparent that non-digestible oligosaccharides and resistant starch also contributed to DF based on their physiological behaviour. In recognition of this, enzymic procedures have been developed to determine these components. A comparison of the dietary fibre contents for a range of food types is given in Table 1.3.
The NSP of foods may be degraded in the gut of pigs by microbial fermentation,
yielding volatile fatty acids, which are absorbed and contribute to the energy supply. A further benefit relates to the volatile fatty acid butyric acid, which is reported
to be an important source of energy for the growth of cells in the epithelium of the
colon; thus, the presence of this acid will promote development of the cells and enhance absorption. The extent of degradation depends on the conformation of the
polymers and their structural association with non-carbohydrate components, such
as lignin. In addition, the physical properties of the NSP, such as water-holding capacity and ion exchange properties, can influence the extent of fermentation. The
gel-forming NSPs, such as -glucan, reduce the absorption of other nutrients from
the small intestine and depress digestibility and adversely affect faecal consistency
in pigs and poultry. On a positive note, the water-holding properties lead to beneficial effects on the behaviour of pregnant sows by increasing time spent eating and
resting owing to increased gut fill and by reducing inappropriate behaviour, such as
bar chewing
 |
| Table 1.3 The fibre components (g/kg dry matter) of some common foods |
Minerals
A simple ash determination provides very little information about the exact mineral
make-up of the food and, when this is required, analytical techniques involving spectroscopy are generally used. In atomic absorption spectroscopy, an acid solution of
the sample is heated in a flame and the vaporised atoms absorb energy, which brings
about transitions from the ground state to higher energy levels.The source of energy
for this transition is a cathode lamp, containing the element to be determined, which
emits radiation at a characteristic wavelength.The radiation absorbed by the atoms in
the flame is proportional to the concentration of the element in the food sample.
Flame emission spectroscopy measures the radiation from solutions of the sample heated in air/acetylene or oxygen/acetylene flames. Each element emits radiation at specific wavelengths and there are published tables of flame emission spectra.
Atomic absorption and flame emission spectrometry are being replaced by inductively coupled plasma emission spectroscopy, as this has a greater sensitivity for the
relatively inert elements and can be used to determine several elements simultaneously or sequentially. Energy from the inductively coupled plasma source is absorbed by argon ions and elements to form a conducting gaseous mixture at
temperatures up to 10 000 °C. The electromagnetic radiation emitted from atoms
and ions within the plasma is then measured.Alternatively the ions can be separated
and detected using a mass spectrometer.
Just as with other nutrients, a measure of the concentration of the element alone
is not sufficient to describe its usefulness to the animal.Attempts have been made to
assess the availability of minerals using chemical methods, such as solubility in water
or dilute acids, but these have had little success. At present animal experiments are
the only reliable way to measure mineral availability
Amino acids, fatty acids and sugars
As an alternative to the standard Kjeldahl method for the determination of nitrogen
(crude protein) described above, the Dumas method is also now used. In this method
the sample is combusted in pure oxygen; the products are carbon dioxide, water, oxides of nitrogen and nitrogen. The carbon dioxide and water are absorbed on
columns and the oxides of nitrogen are converted to nitrogen with a column packed
with copper; the resulting total nitrogen is determined in a thermal conductivity detector.This method, although expensive in equipment, is rapid and does not rely on
hazardous chemicals.
Knowledge of the crude protein content of a food is not a sufficient measure of its
usefulness for non-ruminants.The amino acid composition of the protein is required
in order to assess how a food can meet the essential amino acid requirements (see
Chapter 4). Similarly, the total ether extract content does not give sufficient information on this fraction since it is important to know its fatty acid composition. In nonruminants, this has large effects on the composition of body fat and, if soft fat is to be
avoided, the level of unsaturated fatty acids in the diet must be controlled. In ruminants, a high proportion of unsaturates will depress fibre digestion in the rumen.
When detailed information on the amino acid composition of protein, the fatty acid
composition of fat or the individual sugars in NSP is required, then techniques involving chromatographic separation can be used. In gas–liquid chromatography, the
stationary phase is a liquid held in a porous solid, usually a resin, and the mobile
phase is a gas. Volatile substances partition between the liquid and the vapour and can be effectively isolated. This form of chromatography is, however, usually a slow
process; in order to speed up the separation procedure, high-performance liquid
chromatography has been developed. In this technique, pressure is used to force a
solution, containing the compounds to be separated, rapidly through the resin held
in a strong metal column. In addition to speeding up the process, high resolution is
also obtained. Gas–liquid chromatography and high-performance liquid chromatography can also be used for the determination of certain vitamins (e.g. A, E, B6, K),
but the measurement of available vitamins requires biological methods.
An example of the application of high-performance liquid chromatography is
seen with food proteins, which are hydrolysed with acid and the released amino
acids are then determined using one of the following methods:
- Ion-exchange chromatography – by which the amino acids are separated on the column, and then mixed with a derivatisation agent, which reacts to give a complex that is detected by a spectrophotometer or fluorimeter.
- Reverse-phase chromatography – in which the amino acids react with the reagent to form fluorescent or ultraviolet-absorbing derivatives, which are then separated using a more polar mobile phase (e.g. acetate buffer with a gradient of acetonitrile) and a less polar stationary phase (e.g. octadecyl-bonded silica).The availability of amino acids to the animal can be estimated by chemical methods. For example, for lysine there are colorimetric methods that depend on the formation of compounds between lysine and dyes
Measurement of protein in foods for ruminants
The new methods of expressing the protein requirements of ruminants (see
Chapter 13) require more information than just the crude protein (nitrogen) content
of the food. The unavailable nitrogen is measured as acid detergent insoluble nitrogen. Information on the rate of degradation in the rumen of the available nitrogen is
also required and this can be estimated by biological methods. In the Cornell net carbohydrate and protein system, the neutral and acid detergent extractions of Van Soest,
described above, are used in combination with extraction with a borate–phosphate
buffer and trichloracetic acid solution to derive several protein fractions.These fractions describe the components that are degraded in the rumen or digested in the
small intestine
Spectroscopy
It is now common for laboratories to use near-infrared reflectance spectroscopy
(NIRS) to estimate the composition of foods. The basis of this methodology lies in
the absorption of energy by hydrogen-containing functional groups in organic compounds present in the food (C–H, O–H, N–H and S–H). The reflected energy from
the sample provides information on its composition but, unlike normal spectroscopy,
is not related directly to concentration since the sample is heterogenous. Therefore,
empirical relationships are derived by calibrating the reflected spectrum with samples of known composition, as determined by standard methods. In practice, energy
in the wavelength range 1100–2500 nm is directed on to a cell containing the dried
milled sample, and the diffuse reflected energy is measured across the spectrum.The
spectral data are then related to the known chemical composition of the standard
samples by multiple linear regression. The relationships are then validated with a
second set of samples of known composition. Once satisfactory relationships have
been derived, they can be applied to the spectra of samples of unknown composition.The technique has been extended to the analysis of fresh silage samples, eliminating the need to dry and mill the sample. NIRS has the advantages that it is rapid
with minimal sample preparation, it gives instantaneous results and is non-destructive
of the sample, it allows simultaneous measurement of several parameters with high
precision, and it allows a high throughput of samples at low cost per sample. It is
particularly useful in the context of compound food manufacture where rapid analysis of raw materials and finished product is required for efficient mixing and quality
control standards. With forages, particularly grass and cereal silages, NIRS is now
routinely used to determine not only chemical composition but also a range of food
characteristics, including those that are the resultant of a number of nutrient concentrations such as digestibility, metabolisable energy and nitrogen degradability in the
rumen and potential silage intake (see Chapters 12, 13 and 17).
Nuclear magnetic resonance spectroscopy is a complex technique that is used to
determine the constituents of foods. This method makes use of the fact that some
compounds contain certain atomic nuclei which can be identified from a nuclear
magnetic resonance spectrum, which measures variations in frequency of electromagnetic radiation absorbed. It provides more specific and detailed information of
the conformational structure of compounds than, for example, NIRS but is more
costly and requires more time and skill on the part of the operator. For these
reasons, it is more suited to research work and for cases in which the results from
simpler spectroscopy techniques require further investigation. Nuclear magnetic resonance spectroscopy has been useful in the investigation of the soluble and structural components of forages.
FURTHER READING
Agricultural and Food Research Council 1987 Technical Committee on Responses to Nutrients, report no. 2. Characterisation of feedstuffs: nitrogen. Nutrition Abstracts and
Reviews, Series B: Livestock Feeds and Feeding 57: 713–36.
Agricultural and Food Research Council 1988 Technical Committee on Responses to Nutrients, report no. 3. Characterisation of feedstuffs: other nutrients. Nutrition Abstracts and
Reviews, Series B: Livestock Feeds and Feeding 58: 549–71.
Asp N-G and Johansson C-G 1984 Dietary fibre analysis. Nutrition Abstracts and Reviews 54:
735–51.
Association of Official Analytical Chemists 1990 Official Methods of Analysis, 15th edn,
Washington, DC.
Chalupa W and Sniffen C J 1994 Carbohydrate, protein and amino acid nutrition of lactating
dairy cattle. In: Garnsworthy P C and Cole D J A (eds) Recent Advances in Animal Nutrition, Loughborough, Nottingham University Press, 265–75.
Champ M, Langkilde A-M, Brouns F, Kettlitz B and Le Bail Collet Y 2003 Advances in dietary
fibre characterization. 1. Definition of dietary fibre, physiological relevance, health benefits
and analytical aspects. Nutrition Research Reviews 16: 71–82.
Coultate T P 1989 Food: The Chemistry of its Components, 2nd edn, London, Royal Society
of Chemistry.
Givens D I, De Boever J L and Deaville E R 1997 The principles, practices and some future
applications of near infrared spectroscopy for predicting the nutritive value of foods for
animals and humans. Nutrition Research Reviews 10: 83–114.
Kritchevsky D, Bonfield C and Anderson J W 1988 Dietary Fiber, New York, Plenum Press.
Ministry of Agriculture, Fisheries and Food 1985 The Analysis of Agricultural Materials,
ref. book 427, London, HMSO.
The Feeding Stuffs (Sampling and Analysis) Regulations 1999, London, HMSO.
Van Soest P J 1994 Nutritional Ecology of the Ruminant, 2nd edn, Ithaca, NY, Comstock.







Aucun commentaire:
Enregistrer un commentaire